STERILE DRESSING URGOTUL 15X20 CM 3 PIECES URGO
6% off
€12,88
€13,67
Unit price
/
Unavailable
Tax included, shipping and discounts calculated at checkout.
URGO MEDICAL ITALIA | SKU:
930241979
STERILE DRESSING URGOTUL 15X20 CM 3 PIECES URGO is backordered and will ship as soon as it is back in stock.
Shipping Costs and Times
Shipping Costs and Times
- Order fulfillment within 24 hours and delivery within the following 48/72 hours.
- Shipping cost: always free for orders over €49.90, otherwise it costs €4.99.
Payments
Payments
Payment information is processed securely. We do not store credit card information or have access to your credit card information.
Returns and Refunds
Returns and Refunds
You can return and receive a refund for the item within 30 days. See full policy here.

Do you need help?
Our customer service is here to help you!
- Contact us by phone, email or WhatsApp, from Monday to Friday, from 9:00 to 20:00 .
- For common questions, such as tracking your order or checking its fulfillment status, you can count on our artificial intelligence , available 24/7 .
We are always at your side to offer you fast and effective support!
Description
Description
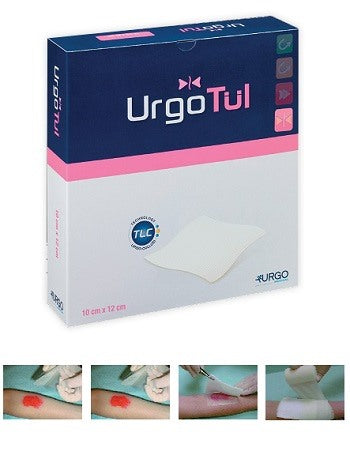
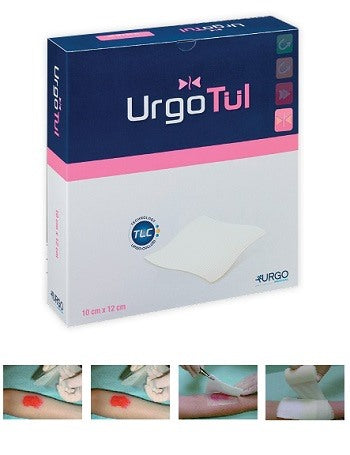
STERILE DRESSING URGOTUL 15X20 CM 3 PIECES URGO
URGO URGOTUL
UrgoTul
Acute and chronic, slightly exuding lesions in the granulation or re-epithelialization phase.
• Healing in a moist environment.
• Atraumatic and painless removal.
• Stimulates the proliferation of fibroblasts.
• Very flexible.
UrgoTul is indicated for the treatment of acute (burns, dermabrasions, traumatic wounds, post-operative wounds, etc.) and chronic (venous ulcers, pressure ulcers, diabetic foot ulcers) lesions in the epithelialization or granulation phase and for the treatment of epidermolysis bullosa lesions.
UrgoTul is available in packs containing individually divided, ready-to-use sterile dressings.
| Dimensions | Unit | Product code | BND Code | BD/RDM |
| 10x12 cm 10x12 cm 15x20 cm 15x20 cm |
3 10 3 10 |
550015 550016 550017 550018 |
M040404 M040404 M040404 M040404 |
287731/R 287731/R 287731/R 287731/R |
Composition
UrgoTul is a non-adhesive, non-occlusive and non-adherent lipid-colloidal dressing, made of a polyester mesh impregnated with hydrocolloidal particles (Carboxymethylcellulose CMC) and Vaseline.
Sterilization mode
Radio-sterilization.
Property
Lipido-Colloidal Technology (TLC) is a healing and gelling matrix, an exclusive and patented innovation from Urgo Laboratories.
Its specificity depends on the polymer matrix which ensures cohesion between the hydrocolloidal particles and the Vaseline in a polyester mesh.
In contact with the wound exudate, the hydrocolloid particles (CMC) gel and form, in association with the matrix, a lipid-colloidal film which allows:
• maintaining a moist environment that is favorable for healing;
• stimulation of fibroblast proliferation.
UrgoTul is greasy in composition, without being greasy to the touch.
It does not adhere to the lesion or to the surrounding skin.
Removal of the dressing is painless for the patient and atraumatic for the regenerating tissues.
Instructions for use
Lesion preparation
• Clean the lesion with saline. If an antiseptic is used first, rinse the lesion thoroughly with saline before applying UrgoTul.
• Dry the surrounding skin thoroughly.
• UrgoTul can be cut using sterile scissors to fit the dressing to the wound.
Application of the dressing
• Remove the protective flaps and apply UrgoTul to the lesion.
• Cover UrgoTul with a secondary dressing and secure with an appropriate bandage.
• Apply a compression bandage if prescribed.
Changing the dressing
• Change the dressing every 2-4 days (every 1-3 days for patients with epidermolysis bullosa). UrgoTul can be left in place for up to 7 days depending on the evolution of the lesion.
Precautions
• Sterile protection must be intact before use. Do not use if sterile protection is damaged.
• If clinical signs of local infection are observed, treatment can be replaced with an antibacterial dressing (UrgoTul Ag/Silver, UrgoCell Ag), according to medical advice.
• In case of deep lesions or fistulas, a part of the UrgoTul dressing should be left visible to allow for easy removal.
• Single-use single-use dressings: reusing a single-use dressing could expose you to the risk of infection.
• Do not re-sterilize.
Status
Medical device, class IIb.