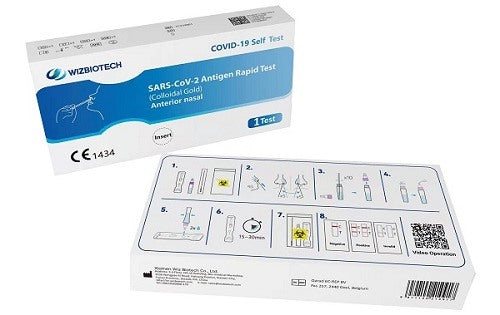
Rapid Antigen Test Covid-19 Self-diagnostic Qualitative Determination of Sars-Cov-2 Antigens in Nasal Swabs by Immunochromatography
91% off
€0,87
€9,90
Unit price
/
Unavailable
Tax included, shipping and discounts calculated at checkout.
DISPOSITIVI ANTI-COVID | SKU:
983776713
Rapid Antigen Test Covid-19 Self-diagnostic Qualitative Determination of Sars-Cov-2 Antigens in Nasal Swabs by Immunochromatography is backordered and will ship as soon as it is back in stock.
Shipping Costs and Times
Shipping Costs and Times
- Order fulfillment within 24 hours and delivery within the following 48/72 hours.
- Shipping cost: always free for orders over €49.90, otherwise it costs €4.99.
Payments
Payments
Payment information is processed securely. We do not store credit card information or have access to your credit card information.
Returns and Refunds
Returns and Refunds
You can return and receive a refund for the item within 30 days. See full policy here.

Do you need help?
Our customer service is here to help you!
- Contact us by phone, email or WhatsApp, from Monday to Friday, from 9:00 to 20:00 .
- For common questions, such as tracking your order or checking its fulfillment status, you can count on our artificial intelligence , available 24/7 .
We are always at your side to offer you fast and effective support!
Description
Description
RAPID ANTIGEN TEST COVID-19 SELF-DIAGNOSTIC QUALITATIVE DETERMINATION OF SARS-COV-2 ANTIGENS IN NASAL SWABS BY IMMUNOCHROMATOGRAPHY
SARS-CoV-2 Antigen Rapid Test
Description
Rapid test intended for the qualitative detection of SARS-CoV-2 antigen (nucleocapsid protein) found in nasal cavity (anterior nasal) specimen from individuals with suspected COVID-19 infection. The test kit is intended for self-testing or home testing.
This test adopts immune lateral chromatography technology. When the test sample contains SARS-CoV-2, the SARS-CoV-2 antigen will react with the antibody coated on the test line (T) to cause a red band to appear in the test line (T) area; when the content of SARS-CoV-2 in the test sample is too low or non-existent, a red band will not appear in the test line (T) area. Regardless of whether the test sample contains SARS-CoV-2, a red band will appear in the quality control line (C) area, which is the basis for judging whether the test is effective.
How to use
1. Use the test kit at room temperature (15°C-30°C). If the kit has been previously stored in a cool place (below 15°C), equilibrate to 15°C-30°C for 30 minutes before testing.
2. Prepare a timer, tissues, hand sanitizer/soap, and warm water.
3. Wash your hands thoroughly (at least 20 seconds) with soap and warm water/hand sanitizer. Then dry your hands.
4. Check the components in the box and make sure there is no damage or breakage.
Sample collection:
1. Remove the sample extraction tube; unscrew the tube cap.
2. Place the extraction tube on the bracket (fixed to the box) to prevent liquid leakage.
3. Tear the swab packaging from the end of the stick and remove the swab.
4. Insert the soft head of the swab into the nostril less than 1 inch; gently rotate the swab against the nasal wall with moderate force at least five times; use the same swab to repeat the collection procedure in the other nostril.
Sample processing:
1. Remove the extraction tube, dip the soft swab head into the extraction solution and immerse it in the liquid.
2. Press the soft head of the swab firmly against the inner wall of the extraction tube and rotate the swab clockwise or counterclockwise approximately 10 times.
3. Push the swab head along the inside wall of the sample extraction tube under the liquid in the tube as far as possible, withdraw the swab.
4. Tighten the standby tube cap.
Sample Test:
1. Tear off the aluminum foil, take out the test card and place it horizontally on the test bench.
2. Disconnect the sample addition hole cover of the extraction tube.
3. Gently squeeze the extraction tube and drop 2 drops of liquid into the sample well of the test card.
4. Start timing, read test results at 15 minutes. Do not read before 15 minutes or after 30 minutes.
5. Upon completion of the test, place all kit materials into the biohazard waste bag and dispose of according to your local biohazard waste disposal policy.
6. Rinse your hands thoroughly for at least 20 seconds with soap and warm water/hand sanitizer.
Negative: The quality control line (C line) appears in the red band, while the test line (T line) does not appear in the red band.
Positive: Both the quality control line (C line) and the test line (T line) appear as red bands.
Warnings
1. For in vitro diagnostic use only.
2. For use with nasal cavity (anterior nasal) swab specimens.
3. Only for the detection of proteins from SARS-CoV-2, not for the detection of other viruses or pathogens.
4. Persons under the age of 18 must be tested with the assistance of a legal guardian or an authorized person.
5. Keep the test kit or kit components out of the reach of children and pets before and after use.
6. The test paper package contains a desiccant, it is forbidden to eat.
7. The sample extraction solution in the extraction tube contains chemical components. Direct contact should be avoided and eating prohibited. If the solution comes into contact with skin, mucous membranes or eyes, rinse with plenty of water. Please contact your GP or a professional or seek medical advice if necessary.
8. The use of gloves and other protective devices is recommended when performing tests.
9. Test kits must be stored according to the storage conditions required in the instructions for use. It is prohibited to use the test kit that has not been stored as required.
10. Do not use the test kit beyond the expiration date.
11. Do not use any kit components that have been opened or modified.
12. Leave the sealed test card in the foil pouch until immediately before use. Do not use if the pouch is damaged or opened.
13. Disposable swabs are sterile products. Do not use if the swab package is damaged or opened.
14. The use of the swab must strictly follow the instructions for use; otherwise it may cause nasal cavity bleeding, swab breakage and retention and other risks.
15. Do not immerse the swab in the extraction solution or other liquid before collecting the specimen with disposable swabs.
16. Do not touch the soft tip of the swab when handling the swab sample.
17. Correct sample collection and handling are essential for correct results.
18. Do not mix components from different kit lots.
19. All kit components are single-use items. Do not use with multiple specimens. Do not reuse the test kit or used kit components.
20. Before deciding to implement relevant therapeutic or management decisions, it is recommended to communicate with family doctors or professionals. Do not take medicines in private or any action that may endanger.
21. Used test components and samples can be placed in plastic bags together with ordinary household waste. If the test result is positive, carefully dispose of the waste components and relevant samples, and thoroughly clean and disinfect the work surface to ensure hygiene. If there are special waste requirements in local laws and regulations, they must be strictly observed.
22. Do not eat, drink, or smoke in the area where specimens or test kits are handled.
Conservation
Store between 2°C and 30°C, in a dry place and away from direct sunlight (do not freeze the kit or its components).
Validity with intact packaging: 12 months.
Validity after opening: 60 minutes.
Format
Main components of the kit:
- Test device.
- Extraction tube.
- Disposable swab.
- Biohazard waste bag.
- Instructions for use.
Rapid test intended for the qualitative detection of SARS-CoV-2 antigen (nucleocapsid protein) found in nasal cavity (anterior nasal) specimen from individuals with suspected COVID-19 infection. The test kit is intended for self-testing or home testing.
This test adopts immune lateral chromatography technology. When the test sample contains SARS-CoV-2, the SARS-CoV-2 antigen will react with the antibody coated on the test line (T) to cause a red band to appear in the test line (T) area; when the content of SARS-CoV-2 in the test sample is too low or non-existent, a red band will not appear in the test line (T) area. Regardless of whether the test sample contains SARS-CoV-2, a red band will appear in the quality control line (C) area, which is the basis for judging whether the test is effective.
How to use
1. Use the test kit at room temperature (15°C-30°C). If the kit has been previously stored in a cool place (below 15°C), equilibrate to 15°C-30°C for 30 minutes before testing.
2. Prepare a timer, tissues, hand sanitizer/soap, and warm water.
3. Wash your hands thoroughly (at least 20 seconds) with soap and warm water/hand sanitizer. Then dry your hands.
4. Check the components in the box and make sure there is no damage or breakage.
Sample collection:
1. Remove the sample extraction tube; unscrew the tube cap.
2. Place the extraction tube on the bracket (fixed to the box) to prevent liquid leakage.
3. Tear the swab packaging from the end of the stick and remove the swab.
4. Insert the soft head of the swab into the nostril less than 1 inch; gently rotate the swab against the nasal wall with moderate force at least five times; use the same swab to repeat the collection procedure in the other nostril.
Sample processing:
1. Remove the extraction tube, dip the soft swab head into the extraction solution and immerse it in the liquid.
2. Press the soft head of the swab firmly against the inner wall of the extraction tube and rotate the swab clockwise or counterclockwise approximately 10 times.
3. Push the swab head along the inside wall of the sample extraction tube under the liquid in the tube as far as possible, withdraw the swab.
4. Tighten the standby tube cap.
Sample Test:
1. Tear off the aluminum foil, take out the test card and place it horizontally on the test bench.
2. Disconnect the sample addition hole cover of the extraction tube.
3. Gently squeeze the extraction tube and drop 2 drops of liquid into the sample well of the test card.
4. Start timing, read test results at 15 minutes. Do not read before 15 minutes or after 30 minutes.
5. Upon completion of the test, place all kit materials into the biohazard waste bag and dispose of according to your local biohazard waste disposal policy.
6. Rinse your hands thoroughly for at least 20 seconds with soap and warm water/hand sanitizer.
Negative: The quality control line (C line) appears in the red band, while the test line (T line) does not appear in the red band.
Positive: Both the quality control line (C line) and the test line (T line) appear as red bands.
Warnings
1. For in vitro diagnostic use only.
2. For use with nasal cavity (anterior nasal) swab specimens.
3. Only for the detection of proteins from SARS-CoV-2, not for the detection of other viruses or pathogens.
4. Persons under the age of 18 must be tested with the assistance of a legal guardian or an authorized person.
5. Keep the test kit or kit components out of the reach of children and pets before and after use.
6. The test paper package contains a desiccant, it is forbidden to eat.
7. The sample extraction solution in the extraction tube contains chemical components. Direct contact should be avoided and eating prohibited. If the solution comes into contact with skin, mucous membranes or eyes, rinse with plenty of water. Please contact your GP or a professional or seek medical advice if necessary.
8. The use of gloves and other protective devices is recommended when performing tests.
9. Test kits must be stored according to the storage conditions required in the instructions for use. It is prohibited to use the test kit that has not been stored as required.
10. Do not use the test kit beyond the expiration date.
11. Do not use any kit components that have been opened or modified.
12. Leave the sealed test card in the foil pouch until immediately before use. Do not use if the pouch is damaged or opened.
13. Disposable swabs are sterile products. Do not use if the swab package is damaged or opened.
14. The use of the swab must strictly follow the instructions for use; otherwise it may cause nasal cavity bleeding, swab breakage and retention and other risks.
15. Do not immerse the swab in the extraction solution or other liquid before collecting the specimen with disposable swabs.
16. Do not touch the soft tip of the swab when handling the swab sample.
17. Correct sample collection and handling are essential for correct results.
18. Do not mix components from different kit lots.
19. All kit components are single-use items. Do not use with multiple specimens. Do not reuse the test kit or used kit components.
20. Before deciding to implement relevant therapeutic or management decisions, it is recommended to communicate with family doctors or professionals. Do not take medicines in private or any action that may endanger.
21. Used test components and samples can be placed in plastic bags together with ordinary household waste. If the test result is positive, carefully dispose of the waste components and relevant samples, and thoroughly clean and disinfect the work surface to ensure hygiene. If there are special waste requirements in local laws and regulations, they must be strictly observed.
22. Do not eat, drink, or smoke in the area where specimens or test kits are handled.
Conservation
Store between 2°C and 30°C, in a dry place and away from direct sunlight (do not freeze the kit or its components).
Validity with intact packaging: 12 months.
Validity after opening: 60 minutes.
Format
Main components of the kit:
- Test device.
- Extraction tube.
- Disposable swab.
- Biohazard waste bag.
- Instructions for use.