COVID-19 FLOWFLEX RAPID ANTIGEN TEST QUALITATIVE DETERMINATION OF SARS-COV-2 ANTIGENS IN NASAL SWABS/SALIVA BY IMMUNOCHROMATOGRAPHY PROFESSIONAL USE
Sold out
€14,90
Unit price
/
Unavailable
Tax included, shipping and discounts calculated at checkout.
Nevia Biotech | SKU:
982504918
Notify me when it's back in stock!
Write us your email, you will be notified when this product becomes available again!
COVID-19 FLOWFLEX RAPID ANTIGEN TEST QUALITATIVE DETERMINATION OF SARS-COV-2 ANTIGENS IN NASAL SWABS/SALIVA BY IMMUNOCHROMATOGRAPHY PROFESSIONAL USE is backordered and will ship as soon as it is back in stock.
Shipping Costs and Times
Shipping Costs and Times
- Order fulfillment within 24 hours and delivery within the following 48/72 hours.
- Shipping cost: always free for orders over €49.90, otherwise it costs €4.99.
Payments
Payments
Payment information is processed securely. We do not store credit card information or have access to your credit card information.
Returns and Refunds
Returns and Refunds
You can return and receive a refund for the item within 30 days. See full policy here.

Do you need help?
Our customer service is here to help you!
- Contact us by phone, email or WhatsApp, from Monday to Friday, from 9:00 to 20:00 .
- For common questions, such as tracking your order or checking its fulfillment status, you can count on our artificial intelligence , available 24/7 .
We are always at your side to offer you fast and effective support!
Description
Description
COVID-19 FLOWFLEX RAPID ANTIGEN TEST QUALITATIVE DETERMINATION OF SARS-COV-2 ANTIGENS IN NASAL SWABS/SALIVA BY IMMUNOCHROMATOGRAPHY PROFESSIONAL USE
Flowflex
Rapid Antigen Test for SARS-CoV-2
(nasal/salivary)
Description
Lateral flow chromatographic immunoassay for the qualitative detection of SARS-CoV-2 virus nucleocapsid protein antigen, in nasal swab or saliva samples, collected directly from individuals with suspected COVID-19 infection by their healthcare provider, within the first seven days of symptom onset.
The test can be used to analyze samples from asymptomatic subjects.
It does not distinguish between SARS-CoV and SARS-CoV-2.
When extracted samples are processed and dispensed into the test cassette, SARS-CoV-2 antigens, if present in the sample, react with the anti-SARS-CoV-2 antibody-coated particles, which are present on the test line (T). The mixture then migrates across the membrane by capillary action. The antigen-conjugated complexes migrate across the test strip to the reaction area and are captured by a line of antibodies bound on the membrane. Test results are interpreted visually after 15 and no later than 30 minutes based on the presence or absence of visible colored lines.
As a unique procedural control, a colored line always appears in the control line region (C) indicating that the correct volume of sample has been added and proper membrane wetting has occurred.
A positive result indicates the presence of the SARS-CoV-2 nucleocapsid antigen. This antigen is usually detectable in upper respiratory tract specimens during the acute phase of infection. As noted, positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine the infection status. Positive results do not rule out bacterial infections or co-infections with other viruses. The agent detected may not be the definitive cause of the disease.
Negative results, in patients with symptoms beyond seven days, should be treated as presumed negative and confirmed with a molecular test for appropriate patient management. Negative results do not exclude SARS-CoV-2 infection and should not be used as the sole guideline for patient treatment or management decisions, including decisions on how to base the clinical approach to the infection. Negative results should be considered in the context of the patient's recent exposures, medical history, and the presence of clinical signs and symptoms consistent with COVID-19.
The test is intended for use by trained clinical laboratory personnel and qualified healthcare professionals at point-of-care facilities.
The SARS-CoV-2 Rapid Antigen Test is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
How to use
The test must be performed immediately after sample collection, or at most within one (1) hour of sample collection, provided that it is stored at room temperature (15-30°C).
The nasal swab sample collection can be performed by a professional or by yourself, but always under the supervision of a healthcare professional.
For children under 12 years of age, specimen collection must be performed by a healthcare professional.
Children aged 12 to 17 years must be supervised by adults if they collect the nasal sample themselves.
Adults (18 years and older) can collect the nasal sample themselves.
Follow local guidelines for specimen collection from children.
How to collect an anterior nasal sample with a swab :
Carefully insert one of the disposable nasal swabs included in the kit into one nostril. Gently twist the swab into the nose no more than 1 inch (2.5 cm) from the end of the nostril. Rub the cotton part of the nasal swab over the inside surface of the nostril (mucosa) using a rotating motion, back and forth, five times in a row. Using the same nasal swab, repeat the procedure in the other nostril to ensure that an adequate sample is collected from both nasal cavities. Remove the swab from the nasal cavity. The sample is now ready for preparation using the tubes containing the extraction buffer solution.
How to collect a saliva sample :
Do not eat, drink, smoke, chew gum, or brush your teeth 30 minutes before collecting a saliva sample.
Before collecting a saliva sample, gently massage your cheeks for 15-30 seconds. Place your tongue against the roots of your lower teeth and allow the saliva to collect there.
Insert a disposable cotton swab (present in the KIT) into your mouth and hold it there for at least 30 seconds, until the tip of the swab is completely saturated with saliva.
Alternatively, saliva can be collected by carefully spitting into the saliva collection container included in the KIT.
When using the saliva collection container to collect saliva, immerse the absorbent tip of the disposable swab into the collection container until the swab tip is completely saturated with saliva.
Note: False negative results may occur if the swab is not completely saturated with saliva.
Allow the sample and extraction buffer solution to reach room temperature (15-30°C) before performing the test.
Use one tube containing Extraction Buffer Solution for each specimen to be tested and label each tube appropriately. Remove the foil from the top of the Extraction Buffer Solution tube. Insert the swab into the tube and swirl for 30 seconds. Then swirl the swab at least 5 times while squeezing the sides of the tube. Be careful not to splash the contents out of the tube. Remove the swab by squeezing the sides of the tube to extract liquid from the swab. Firmly attach the dropper tip to the Extraction Buffer Solution tube containing the specimen. Mix thoroughly by swirling and tapping the bottom of the tube. Remove the test cassette from the foil pouch and use as soon as possible. Place the test cassette on a clean, flat surface. Add the processed specimen to the appropriate well of the test cassette. Invert the tube containing Extraction Buffer Solution and specimen with the dropper tip facing down, keeping it upright. Gently squeeze the tube, pouring 4 drops of the treated sample into the sampling well.
Wait for the colored lines to develop. The result should be read after 15-30 minutes. Do not read the result after 30 minutes.
Interpretation of results :
- NEGATIVE: Only one colored line appears in the control line region (C). No visible colored line appears in the test line region (T). This means that no SARS-CoV-2 antigen was detected.
- POSITIVE: *Two distinct colored lines appear. One line in the control line region (C) and the other in the test line region (T). This means that SARS-CoV-2 antigen has been detected.
- INVALID: No control line appears. Insufficient sample volume or incorrect usage are the most likely reasons for the control line not appearing. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue use of the kit and contact your local distributor.
*NOTE: The color intensity of the test line (T) may vary depending on the level of SARS-CoV-2 antigen present in the specimen. Therefore, any shade of color in the test line (T) region should be considered positive.
Warnings
For professional in vitro diagnostic use only. Do not use beyond expiration date.
Do not eat, drink or smoke in the area where the specimens or kit are handled.
Do not use the test if the outer wrapper is damaged.
Handle all specimens as if they contain infectious agents. Observe established biohazard precautions during testing and follow standard procedures for proper specimen disposal.
Wear protective clothing, such as lab coats, disposable gloves, masks, and eye protection when testing samples.
The used test should be considered potentially infectious and disposed of in accordance with local regulations.
Humidity and temperature can negatively affect results.
Read the package leaflet carefully before performing the test. Failure to follow the instructions in the package leaflet may result in inaccurate test results.
The test line for a sample with a high viral load may become visible within 15 minutes, or as soon as the sample passes the test line area.
The test line for a sample with a low viral load becomes visible after 30 minutes.
The SARS-CoV-2 Rapid Antigen Test is intended for in vitro diagnostic use only. The test is intended for the detection of SARS-CoV-2 virus antigens in nasal swab or saliva specimens. The intensity of the test line does not necessarily correlate with the SARS-CoV-2 viral titer present in the specimen. Please note that the viral load in saliva specimen is relatively low, so it is recommended to use a nasal specimen. If nasal sampling is not possible, saliva specimen may be used.
Samples must be tested as quickly as possible after sample collection and no later than one hour after collection.
The use of storage and stabilization media (media) for specimen transport may result in a reduction in test sensitivity.
A false negative test may occur if the antigen concentration in a sample is below the test's detection limit or if the sample was collected incorrectly.
Test results should be correlated with other clinical data available to the physician.
A positive test result does not exclude co-infections with other pathogens.
A positive test result does not distinguish between SARS-CoV and SARS-CoV-2. A negative test result is not intended to rule out other viral or bacterial infections. A negative test result in a patient with onset of symptoms beyond seven days should be treated as presumed negative and confirmed with a molecular test, based on which clinical treatment should be determined (if differentiation of specific SARS viruses and strains is necessary, additional testing will be required.)
Conservation
The kit can be stored at temperatures between 2-30°C. Do not freeze.
The test is stable until the expiration date printed on the sealed outer pouch. Do not use beyond the expiration date.
The test must remain in the sealed pouch until use.
Validity with intact packaging: 24 months.
Format
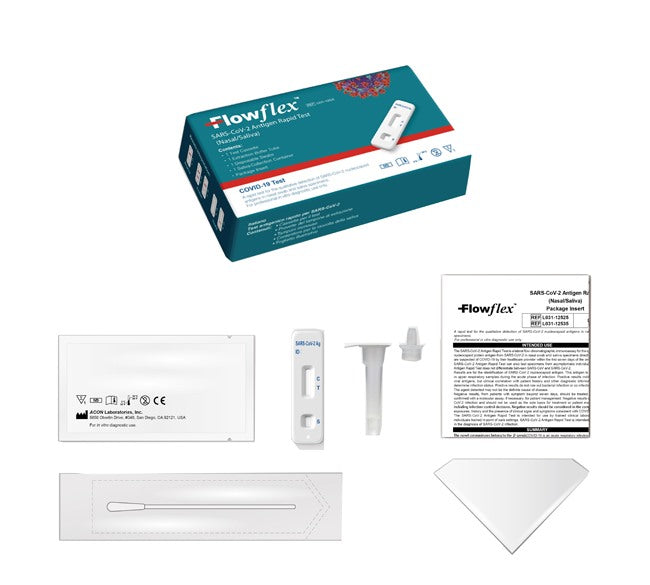
The kit contains:
- 1 test cassette;
- 1 disposable swab (made by another manufacturer);
- 1 leaflet;
- 1 test tube with extraction buffer solution;
- 1 container for saliva collection.
Bibliography
1. Shuo Su, Gary Wong, Weifeng Shi, et al. Epidemiology, Genetic recombination, and pathogenesis of coronaviruses. Trends in Microbiology, June 2016, vol. 24, No. 6: 490-502
2. Susan R. Weiss, Julian L. Leibowitz, Coronavirus Pathogenesis, Advances in Virus Research, Volume 81: 85-164
Cod. L031-12525
Lateral flow chromatographic immunoassay for the qualitative detection of SARS-CoV-2 virus nucleocapsid protein antigen, in nasal swab or saliva samples, collected directly from individuals with suspected COVID-19 infection by their healthcare provider, within the first seven days of symptom onset.
The test can be used to analyze samples from asymptomatic subjects.
It does not distinguish between SARS-CoV and SARS-CoV-2.
When extracted samples are processed and dispensed into the test cassette, SARS-CoV-2 antigens, if present in the sample, react with the anti-SARS-CoV-2 antibody-coated particles, which are present on the test line (T). The mixture then migrates across the membrane by capillary action. The antigen-conjugated complexes migrate across the test strip to the reaction area and are captured by a line of antibodies bound on the membrane. Test results are interpreted visually after 15 and no later than 30 minutes based on the presence or absence of visible colored lines.
As a unique procedural control, a colored line always appears in the control line region (C) indicating that the correct volume of sample has been added and proper membrane wetting has occurred.
A positive result indicates the presence of the SARS-CoV-2 nucleocapsid antigen. This antigen is usually detectable in upper respiratory tract specimens during the acute phase of infection. As noted, positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine the infection status. Positive results do not rule out bacterial infections or co-infections with other viruses. The agent detected may not be the definitive cause of the disease.
Negative results, in patients with symptoms beyond seven days, should be treated as presumed negative and confirmed with a molecular test for appropriate patient management. Negative results do not exclude SARS-CoV-2 infection and should not be used as the sole guideline for patient treatment or management decisions, including decisions on how to base the clinical approach to the infection. Negative results should be considered in the context of the patient's recent exposures, medical history, and the presence of clinical signs and symptoms consistent with COVID-19.
The test is intended for use by trained clinical laboratory personnel and qualified healthcare professionals at point-of-care facilities.
The SARS-CoV-2 Rapid Antigen Test is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
How to use
The test must be performed immediately after sample collection, or at most within one (1) hour of sample collection, provided that it is stored at room temperature (15-30°C).
The nasal swab sample collection can be performed by a professional or by yourself, but always under the supervision of a healthcare professional.
For children under 12 years of age, specimen collection must be performed by a healthcare professional.
Children aged 12 to 17 years must be supervised by adults if they collect the nasal sample themselves.
Adults (18 years and older) can collect the nasal sample themselves.
Follow local guidelines for specimen collection from children.
How to collect an anterior nasal sample with a swab :
Carefully insert one of the disposable nasal swabs included in the kit into one nostril. Gently twist the swab into the nose no more than 1 inch (2.5 cm) from the end of the nostril. Rub the cotton part of the nasal swab over the inside surface of the nostril (mucosa) using a rotating motion, back and forth, five times in a row. Using the same nasal swab, repeat the procedure in the other nostril to ensure that an adequate sample is collected from both nasal cavities. Remove the swab from the nasal cavity. The sample is now ready for preparation using the tubes containing the extraction buffer solution.
How to collect a saliva sample :
Do not eat, drink, smoke, chew gum, or brush your teeth 30 minutes before collecting a saliva sample.
Before collecting a saliva sample, gently massage your cheeks for 15-30 seconds. Place your tongue against the roots of your lower teeth and allow the saliva to collect there.
Insert a disposable cotton swab (present in the KIT) into your mouth and hold it there for at least 30 seconds, until the tip of the swab is completely saturated with saliva.
Alternatively, saliva can be collected by carefully spitting into the saliva collection container included in the KIT.
When using the saliva collection container to collect saliva, immerse the absorbent tip of the disposable swab into the collection container until the swab tip is completely saturated with saliva.
Note: False negative results may occur if the swab is not completely saturated with saliva.
Allow the sample and extraction buffer solution to reach room temperature (15-30°C) before performing the test.
Use one tube containing Extraction Buffer Solution for each specimen to be tested and label each tube appropriately. Remove the foil from the top of the Extraction Buffer Solution tube. Insert the swab into the tube and swirl for 30 seconds. Then swirl the swab at least 5 times while squeezing the sides of the tube. Be careful not to splash the contents out of the tube. Remove the swab by squeezing the sides of the tube to extract liquid from the swab. Firmly attach the dropper tip to the Extraction Buffer Solution tube containing the specimen. Mix thoroughly by swirling and tapping the bottom of the tube. Remove the test cassette from the foil pouch and use as soon as possible. Place the test cassette on a clean, flat surface. Add the processed specimen to the appropriate well of the test cassette. Invert the tube containing Extraction Buffer Solution and specimen with the dropper tip facing down, keeping it upright. Gently squeeze the tube, pouring 4 drops of the treated sample into the sampling well.
Wait for the colored lines to develop. The result should be read after 15-30 minutes. Do not read the result after 30 minutes.
Interpretation of results :
- NEGATIVE: Only one colored line appears in the control line region (C). No visible colored line appears in the test line region (T). This means that no SARS-CoV-2 antigen was detected.
- POSITIVE: *Two distinct colored lines appear. One line in the control line region (C) and the other in the test line region (T). This means that SARS-CoV-2 antigen has been detected.
- INVALID: No control line appears. Insufficient sample volume or incorrect usage are the most likely reasons for the control line not appearing. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue use of the kit and contact your local distributor.
*NOTE: The color intensity of the test line (T) may vary depending on the level of SARS-CoV-2 antigen present in the specimen. Therefore, any shade of color in the test line (T) region should be considered positive.
Warnings
For professional in vitro diagnostic use only. Do not use beyond expiration date.
Do not eat, drink or smoke in the area where the specimens or kit are handled.
Do not use the test if the outer wrapper is damaged.
Handle all specimens as if they contain infectious agents. Observe established biohazard precautions during testing and follow standard procedures for proper specimen disposal.
Wear protective clothing, such as lab coats, disposable gloves, masks, and eye protection when testing samples.
The used test should be considered potentially infectious and disposed of in accordance with local regulations.
Humidity and temperature can negatively affect results.
Read the package leaflet carefully before performing the test. Failure to follow the instructions in the package leaflet may result in inaccurate test results.
The test line for a sample with a high viral load may become visible within 15 minutes, or as soon as the sample passes the test line area.
The test line for a sample with a low viral load becomes visible after 30 minutes.
The SARS-CoV-2 Rapid Antigen Test is intended for in vitro diagnostic use only. The test is intended for the detection of SARS-CoV-2 virus antigens in nasal swab or saliva specimens. The intensity of the test line does not necessarily correlate with the SARS-CoV-2 viral titer present in the specimen. Please note that the viral load in saliva specimen is relatively low, so it is recommended to use a nasal specimen. If nasal sampling is not possible, saliva specimen may be used.
Samples must be tested as quickly as possible after sample collection and no later than one hour after collection.
The use of storage and stabilization media (media) for specimen transport may result in a reduction in test sensitivity.
A false negative test may occur if the antigen concentration in a sample is below the test's detection limit or if the sample was collected incorrectly.
Test results should be correlated with other clinical data available to the physician.
A positive test result does not exclude co-infections with other pathogens.
A positive test result does not distinguish between SARS-CoV and SARS-CoV-2. A negative test result is not intended to rule out other viral or bacterial infections. A negative test result in a patient with onset of symptoms beyond seven days should be treated as presumed negative and confirmed with a molecular test, based on which clinical treatment should be determined (if differentiation of specific SARS viruses and strains is necessary, additional testing will be required.)
Conservation
The kit can be stored at temperatures between 2-30°C. Do not freeze.
The test is stable until the expiration date printed on the sealed outer pouch. Do not use beyond the expiration date.
The test must remain in the sealed pouch until use.
Validity with intact packaging: 24 months.
Format
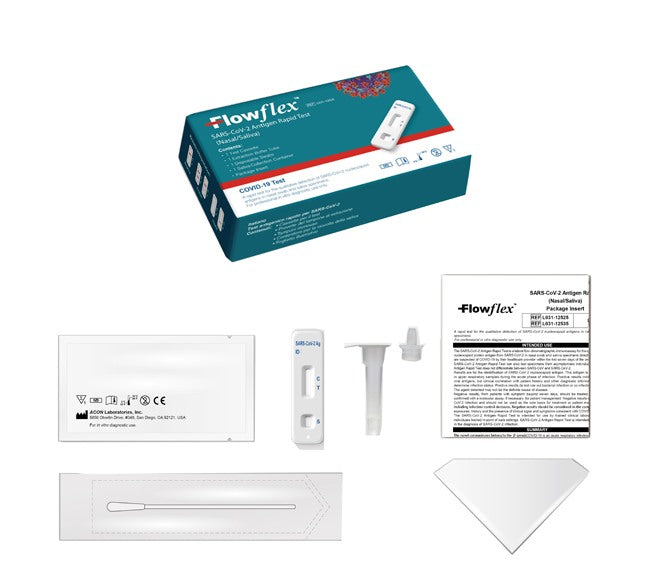
The kit contains:
- 1 test cassette;
- 1 disposable swab (made by another manufacturer);
- 1 leaflet;
- 1 test tube with extraction buffer solution;
- 1 container for saliva collection.
Bibliography
1. Shuo Su, Gary Wong, Weifeng Shi, et al. Epidemiology, Genetic recombination, and pathogenesis of coronaviruses. Trends in Microbiology, June 2016, vol. 24, No. 6: 490-502
2. Susan R. Weiss, Julian L. Leibowitz, Coronavirus Pathogenesis, Advances in Virus Research, Volume 81: 85-164
Cod. L031-12525